The Medicines Control Agency (MCA), the national drug regulatory body of Gambia, has recently informed the drug authorities in India that it is introducing a regulation under which all imported pharmaceutical products will be inspected and samples for testing should ensure conformity to quality standards prior to shipment from India. In a letter issued to the Drugs Controller General of India (DCGI), Dr Rajeev Singh Raghuvanshi, on June 15, 2023, the MCA informed the DCGI that it is introducing a regulation of pre-shipment document verification, physical inspection, quality control testing and issuance of Clean Report of Inspection and Analysis (CRIA) for the pharma exports to the Gambia to address the issues related to substandard and falsified or counterfeit medicines entering the country. The MCA has now appointed Mumbai-based Quntrol Laboratories Pvt Ltd, an independent verification, inspection and testing company for pharmaceuticals, to carry out the process and issue CRIA for all shipments. The importer shall require a CRIA issued by Quntrol to clear their goods at the Ports of Entry in the Gambia. The new regulation has come into force mandatorily from July 1, 2023. The Gambian move comes after the health authorities in the Gambia and the World Health Organisation alleged last year that four cough syrups manufactured in India, for which the manufacturer is Haryana-based Maiden Pharmaceuticals, were linked with acute kidney injuries and death of 66 children in the West African country. The Gambian drug authorities have categorically mentioned that without this mandatory CRIA document, goods will not be accepted in the importation process and if conformity is established at all levels, Quntrol will issue mandatory CRIA documents and if conformity is not established with regards to quality of the product, the shipment will be quarantined or seized by the MCA and the necessary regulatory actions shall be taken.
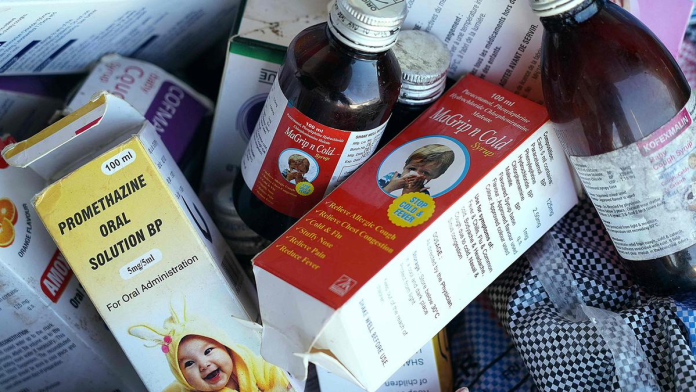
Quite obviously, the Gambian drug authority’s action in this regard is quite understandable as earlier last year, the Indian pharmaceutical industry’s image was severely beaten when the WHO issued alert against four contaminated medicines – Promethazine oral solution, Kofexmalin baby cough syrup, Makoff baby cough syrup and Magrip N cold syrup – manufactured by the Haryana-based Maiden Pharmaceuticals that resulted in the death of 66 children in Gambia. Later last year, the Department of Drug Administration, Nepal had published a list of 16 manufacturing facilities of Indian pharmaceutical companies for failing to comply with the WHO’s Good Manufacturing Practices (WHO-GMP). The list of facilities which were not complied with the WHO-GMP standards included several big names in the industry. Clearly, the introduction of a new regulation by MCA is a wake-up call for the Indian drug authorities to consistently maintain the quality of pharmaceutical products manufactured by Indian companies. It is high time the drug authorities take the issue seriously and rectify the errors in the regulatory framework to ensure that only quality products are exported from the country. Though the Indian drug authorities have taken some actions, it should further intensify its quality systems in manufacture and in-house testing of drugs with stringent adherence to good laboratory practices (GLP). As the country’s pharmaceutical industry’s image has taken a severe beating during the last couple of years, there is pressing need to increase monitoring of WHO-GMP drug manufacturing units by the State Licensing Authorities (SLAs) in the country as it is the SLAs who issue Certificate of Pharmaceutical Product (CoPP) to the drug manufacturing units under the WHO-GMP Certification Scheme for the purpose of international commerce.